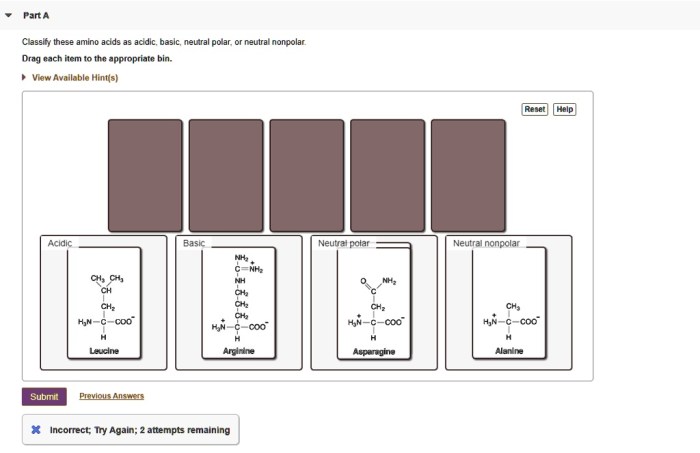
Classify these amino acids as acidic basic neutral polar – In the realm of biochemistry, classifying amino acids as acidic, basic, neutral, polar, and nonpolar is a fundamental concept that unveils the intricate nature of these building blocks of proteins. This classification hinges upon the properties of their side chains, which determine their behavior in various chemical environments and ultimately shape the structure and function of proteins.
Delving into the nuances of amino acid classification, we will explore the defining characteristics of each group, unraveling the intricate interplay between their chemical properties and their biological roles.
Amino Acid Properties: Classify These Amino Acids As Acidic Basic Neutral Polar
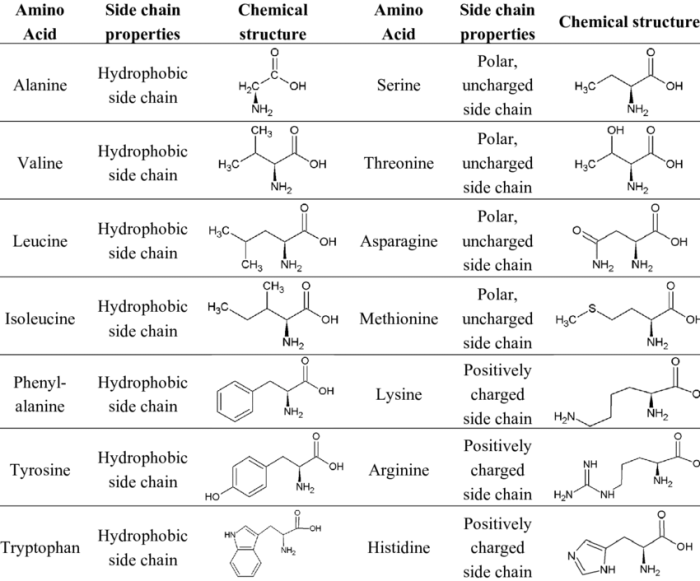
Amino acids are the building blocks of proteins. They have a central carbon atom bonded to an amino group, a carboxylic acid group, a hydrogen atom, and a side chain.
The side chain is what distinguishes one amino acid from another. It can be anything from a simple hydrogen atom to a complex organic molecule.
The 20 common amino acids are listed in the table below. Their abbreviations are one or three letters long. The side chain properties are given in the third column.
| Amino Acid | Abbreviation | Side Chain Property |
|---|---|---|
| Alanine | Ala | Nonpolar |
| Arginine | Arg | Basic |
| Asparagine | Asn | Polar |
| Aspartic acid | Asp | Acidic |
| Cysteine | Cys | Polar |
| Glutamic acid | Glu | Acidic |
| Glutamine | Gln | Polar |
| Glycine | Gly | Nonpolar |
| Histidine | His | Basic |
| Isoleucine | Ile | Nonpolar |
| Leucine | Leu | Nonpolar |
| Lysine | Lys | Basic |
| Methionine | Met | Nonpolar |
| Phenylalanine | Phe | Nonpolar |
| Proline | Pro | Nonpolar |
| Glutamine | Gln | Polar |
| Serine | Ser | Polar |
| Threonine | Thr | Polar |
| Tryptophan | Trp | Nonpolar |
| Tyrosine | Tyr | Polar |
| Valine | Val | Nonpolar |
Amino Acid Classification
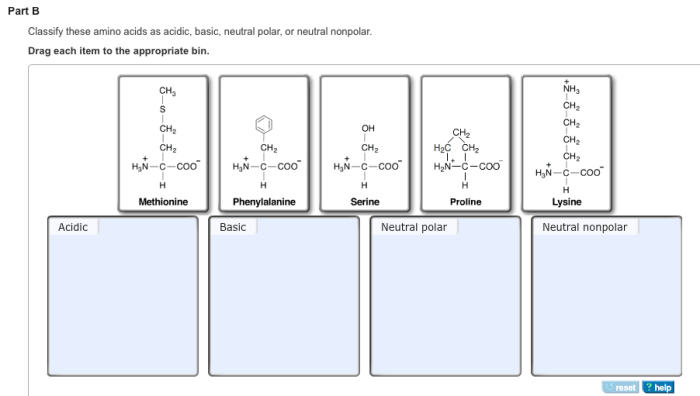
Acidic Amino Acids, Classify these amino acids as acidic basic neutral polar
Acidic amino acids have a net negative charge at physiological pH. They have a carboxylic acid group in their side chain.
The acidic amino acids are aspartic acid and glutamic acid.
Basic Amino Acids
Basic amino acids have a net positive charge at physiological pH. They have an amino group in their side chain.
The basic amino acids are arginine, histidine, and lysine.
Neutral Amino Acids
Neutral amino acids have no net charge at physiological pH. They may have polar or nonpolar side chains.
The neutral amino acids are alanine, glycine, isoleucine, leucine, methionine, phenylalanine, proline, serine, threonine, tryptophan, tyrosine, and valine.
| Amino Acid | Classification |
|---|---|
| Alanine | Neutral |
| Arginine | Basic |
| Asparagine | Polar |
| Aspartic acid | Acidic |
| Cysteine | Polar |
| Glutamic acid | Acidic |
| Glutamine | Polar |
| Glycine | Neutral |
| Histidine | Basic |
| Isoleucine | Neutral |
| Leucine | Neutral |
| Lysine | Basic |
| Methionine | Neutral |
| Phenylalanine | Neutral |
| Proline | Neutral |
| Glutamine | Polar |
| Serine | Polar |
| Threonine | Polar |
| Tryptophan | Neutral |
| Tyrosine | Polar |
| Valine | Neutral |
Polar and Nonpolar Amino Acids
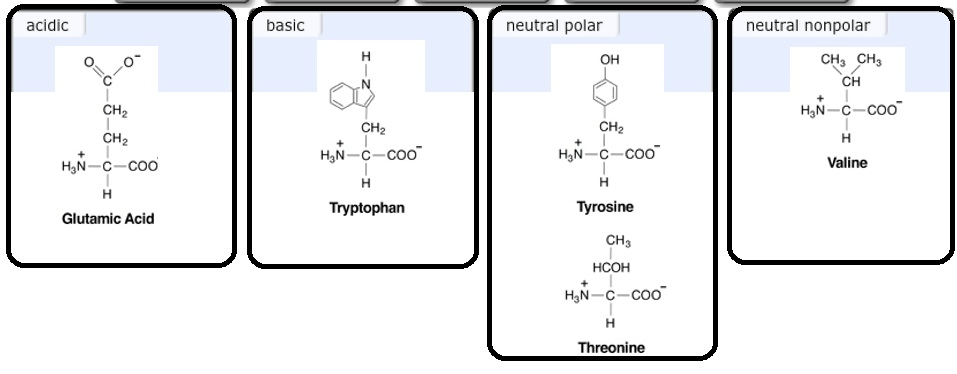
Polar Amino Acids
Polar amino acids have side chains that can form hydrogen bonds with water. They are hydrophilic, meaning they are attracted to water.
The polar amino acids are asparagine, glutamine, serine, threonine, tyrosine, and tryptophan.
Nonpolar Amino Acids
Nonpolar amino acids have side chains that cannot form hydrogen bonds with water. They are hydrophobic, meaning they are repelled by water.
The nonpolar amino acids are alanine, glycine, isoleucine, leucine, methionine, phenylalanine, proline, tryptophan, and valine.
| Amino Acid | Classification |
|---|---|
| Alanine | Nonpolar |
| Arginine | Basic |
| Asparagine | Polar |
| Aspartic acid | Acidic |
| Cysteine | Polar |
| Glutamic acid | Acidic |
| Glutamine | Polar |
| Glycine | Nonpolar |
| Histidine | Basic |
| Isoleucine | Nonpolar |
| Leucine | Nonpolar |
| Lysine | Basic |
| Methionine | Nonpolar |
| Phenylalanine | Nonpolar |
| Proline | Nonpolar |
| Glutamine | Polar |
| Serine | Polar |
| Threonine | Polar |
| Tryptophan | Nonpolar |
| Tyrosine | Polar |
| Valine | Nonpolar |
Amino Acid Interactions
Amino acids can interact with each other in a variety of ways. These interactions include:
- Covalent bonds
- Hydrogen bonds
- Ionic bonds
- Van der Waals forces
These interactions contribute to the structure and function of proteins. For example, covalent bonds hold the amino acids together in a polypeptide chain, while hydrogen bonds and ionic bonds help to stabilize the protein’s structure.
Amino acid interactions can also be used to design new drugs and materials. For example, drugs that target specific amino acids can be used to treat diseases. Similarly, materials that are made from amino acids can be used to create new products with unique properties.
Q&A
What is the significance of classifying amino acids?
Classifying amino acids allows us to understand their chemical properties and how they interact with each other, which is crucial for comprehending protein structure and function.
How does the side chain of an amino acid influence its classification?
The side chain of an amino acid determines its charge and polarity, which are the key factors in classifying it as acidic, basic, neutral, polar, or nonpolar.
Why is it important to consider the polarity of amino acids?
The polarity of amino acids influences their solubility and interactions with water, which is essential for understanding protein folding and function.