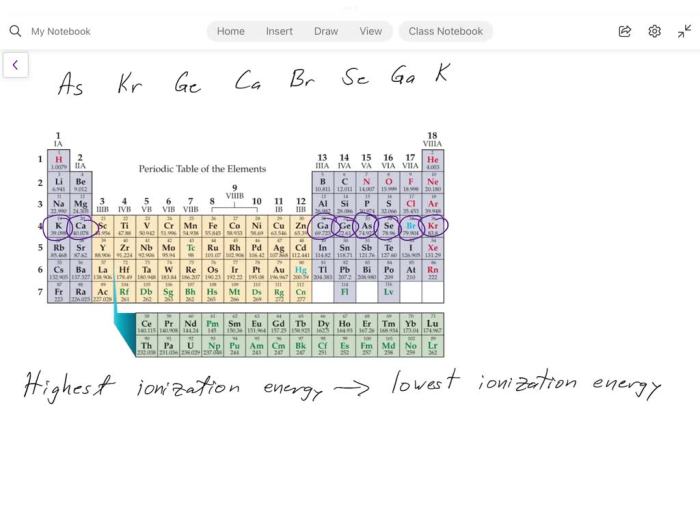
Rank the following elements by ionization energy – The concept of ionization energy, a fundamental property of elements, takes center stage in this comprehensive guide. As we delve into the factors influencing ionization energy and explore its periodic trends, we will uncover its practical applications and delve into the techniques used to determine it experimentally and theoretically.
Ionization Energy Definition and Concept
Ionization energy refers to the minimum amount of energy required to remove an electron from a neutral atom or ion in its gaseous state. It is a fundamental property of elements that provides insights into their atomic structure and chemical reactivity.
For example, the ionization energy of hydrogen is 1312 kJ/mol, which means that 1312 kJ of energy is needed to remove an electron from a hydrogen atom.
Factors Affecting Ionization Energy
Atomic Radius
Ionization energy generally decreases with increasing atomic radius. This is because electrons in larger atoms are further away from the nucleus, experiencing a weaker attractive force. As a result, it requires less energy to remove an electron from a larger atom.
Nuclear Charge
Ionization energy increases with increasing nuclear charge. A higher nuclear charge exerts a stronger attractive force on the electrons, making it more difficult to remove an electron.
Electron Configuration, Rank the following elements by ionization energy
Electron configuration plays a significant role in ionization energy. Atoms with stable electron configurations, such as noble gases, have higher ionization energies compared to atoms with unstable configurations.
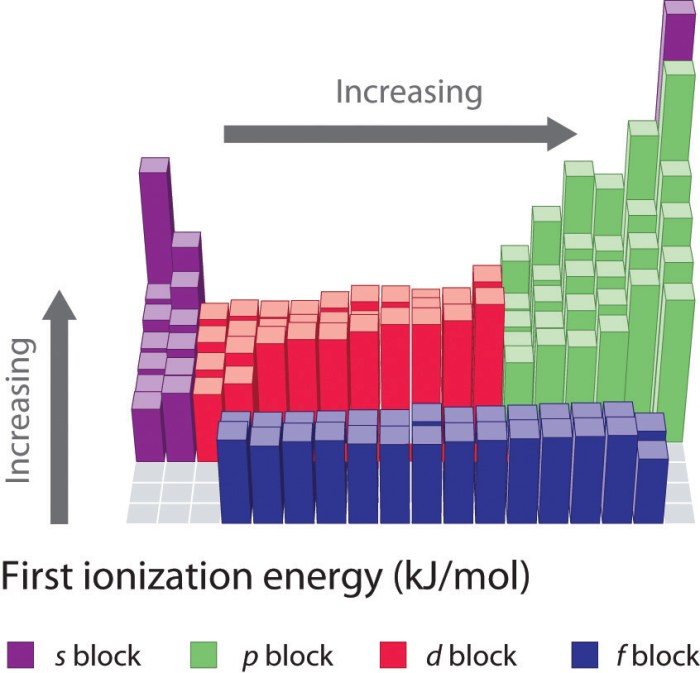
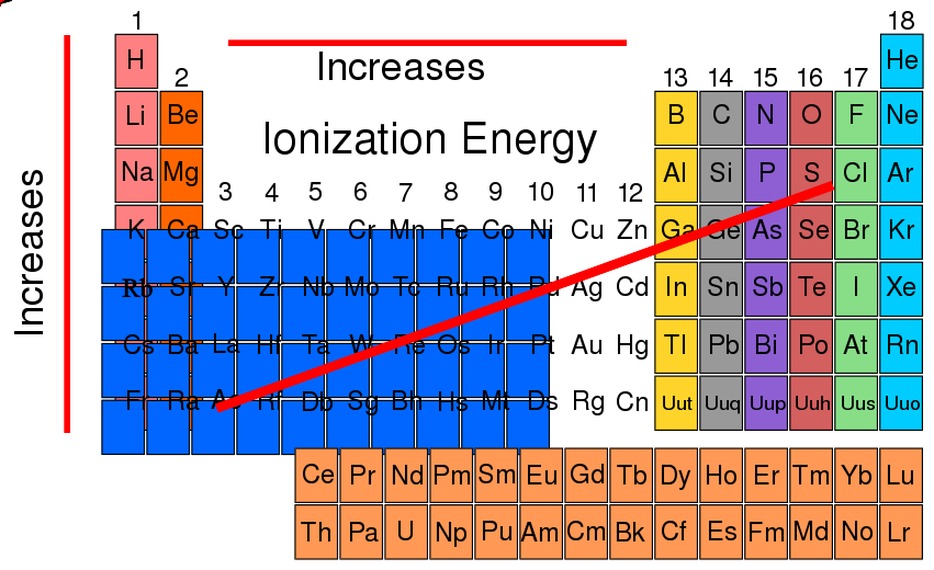
Trends in Ionization Energy
Ionization energy exhibits periodic trends across the periodic table:
- Across a period:Ionization energy generally increases from left to right as the atomic number increases. This is due to the increase in nuclear charge.
- Down a group:Ionization energy generally decreases from top to bottom within a group. This is because the atomic radius increases down a group, leading to a weaker attraction between the nucleus and electrons.
Applications of Ionization Energy
Ionization energy has practical applications in various fields:
- Chemistry:Ionization energy helps determine atomic properties, predict chemical reactivity, and design new materials.
- Physics:Ionization energy is used in the study of atomic and molecular physics, including plasma physics and spectroscopy.
- Materials Science:Ionization energy is considered in the development of semiconductors, superconductors, and other advanced materials.
Experimental Determination of Ionization Energy: Rank The Following Elements By Ionization Energy
Ionization energy can be experimentally determined using techniques such as:
- Mass Spectrometry:Measures the mass-to-charge ratio of ions, providing information about the energy required to remove electrons.
- Atomic Emission Spectroscopy:Analyzes the light emitted by excited atoms, which corresponds to the energy difference between excited and ionized states.
Theoretical Calculations of Ionization Energy
Ionization energy can also be calculated theoretically using methods such as:
- Hartree-Fock Method:Approximates the wave function of electrons in an atom, providing an estimate of ionization energy.
- Density Functional Theory:A more advanced method that considers the electron density distribution to calculate ionization energy.
FAQ Insights
What is the definition of ionization energy?
Ionization energy is the minimum energy required to remove an electron from an atom or ion in its gaseous state.
Which factors affect ionization energy?
Ionization energy is primarily influenced by atomic radius, nuclear charge, and electron configuration.
How does ionization energy vary across the periodic table?
Ionization energy generally increases from left to right across a period and decreases from top to bottom within a group.