The reaction shown is of what type – Chemical reactions are the driving force behind countless phenomena in our world, from the combustion of fuels to the digestion of food. Understanding the type of reaction that is occurring is crucial for predicting its behavior and potential applications. In this exploration, we delve into the fascinating realm of chemical reaction types, unraveling the intricacies of combination, decomposition, single-replacement, double-replacement, and combustion reactions.
Through a comprehensive analysis of reaction mechanisms, we uncover the fundamental processes that govern chemical transformations. We examine the role of catalysts and inhibitors in accelerating or decelerating reactions, respectively, and explore the energy changes that accompany these processes, distinguishing between exothermic and endothermic reactions.
Chemical Reaction Types
Chemical reactions are processes that involve the transformation of one set of chemical substances (reactants) into another set of chemical substances (products). There are several types of chemical reactions, each characterized by its unique pattern of reactant and product interactions.
Combination Reactions
Combination reactions, also known as synthesis reactions, involve the combination of two or more reactants to form a single product. For example:
H2+ O 2→ 2H 2O
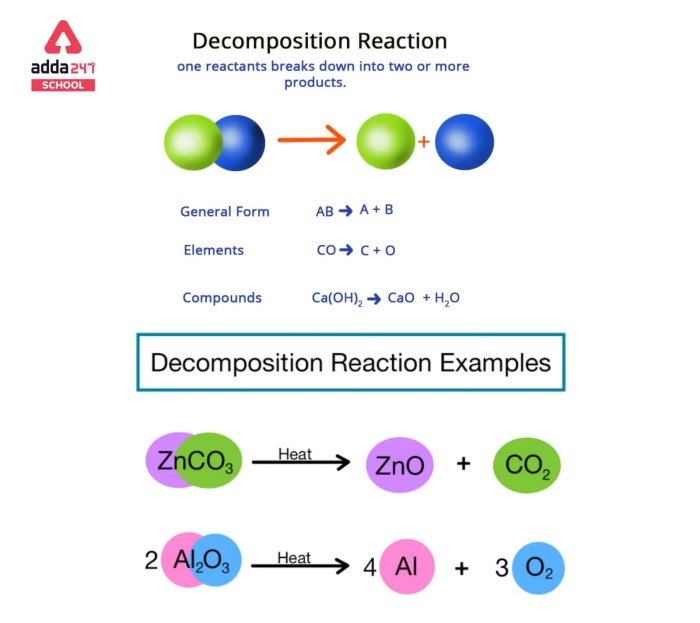
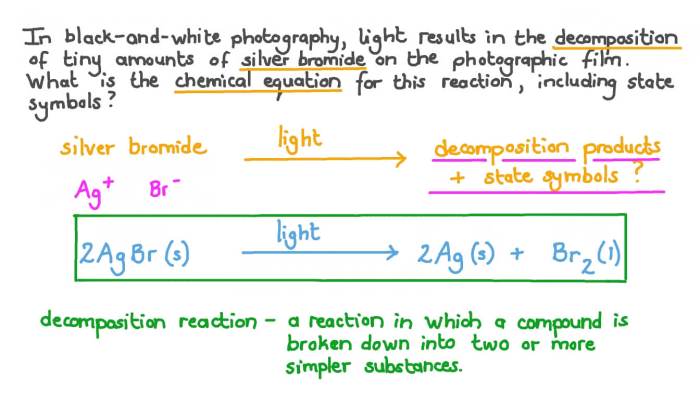
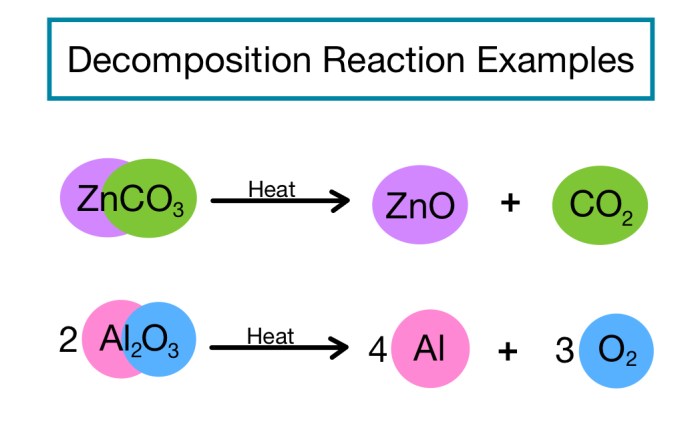
Decomposition Reactions
Decomposition reactions involve the breakdown of a single reactant into two or more products. For example:
H2O → 2H 2+ O 2
Single-Replacement Reactions, The reaction shown is of what type
Single-replacement reactions involve the replacement of one element in a compound by another element. For example:
Zn + 2HCl → ZnCl2+ H 2
Double-Replacement Reactions
Double-replacement reactions involve the exchange of ions between two compounds. For example:
AgNO3+ NaCl → AgCl + NaNO 3
Combustion Reactions
Combustion reactions involve the reaction of a substance with oxygen, typically resulting in the production of heat and light. For example:
CH4+ 2O 2→ CO 2+ 2H 2O
Question Bank: The Reaction Shown Is Of What Type
What are the key factors that influence the rate of a chemical reaction?
The rate of a chemical reaction is primarily determined by factors such as concentration of reactants, temperature, surface area, and the presence of catalysts or inhibitors.
How can we predict the equilibrium position of a chemical reaction?
The equilibrium position of a reaction can be predicted by considering factors such as temperature, pressure, and the initial concentrations of reactants and products.
What are some practical applications of chemical reactions in everyday life?
Chemical reactions play a vital role in numerous aspects of our daily lives, including the production of food, pharmaceuticals, and energy, as well as in environmental processes such as pollution control and water purification.